Decomposition mechanism of arsine on GaAs (001)-(4x2)
Arsine has been extensively used in MOCVD growth of
gallium arsenide-based electronic devices. To improve the epitaxy
growth process, one needs to understand the adsorption and decomposition
mechanism of AsH3 molecule on the GaAs (001) surface.
By using infrared spectroscopy, scanning tunneling microscopy, and
ab initio molecular calculation, we have determined the sequential
decomposition mechanism of AsH3 on the gallium-rich GaAs (4x2)
surface. Particularly, we have found that the the rate-limiting step
of AsH3 dissociation is the initial cleavage of As-H bond and
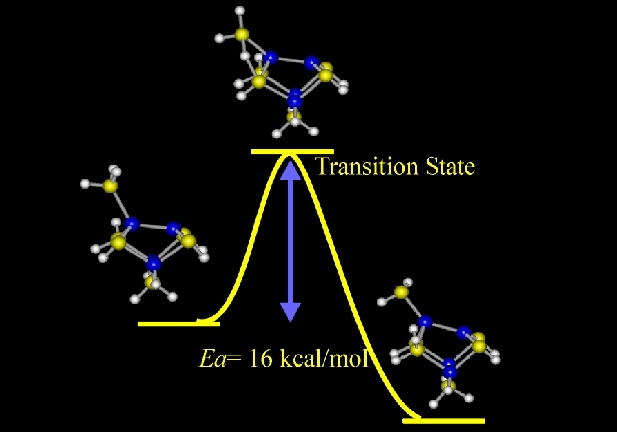
transfer of H to the second-layer arsenic-site. Shown above are the
molecular models for this elemental reaction. The theoretical
calculation found that an activation energy of 16 kcal/mol has to be
overcome in order for this reaction to proceed. This result is in
excellent agreement with the kinetic analysis of isotherms obtained from
experiment.
This work has been recently reported on J. Phys. Chem. B
© Copyright 1996-2007, R. F. Hicks, Semiconductor Material Chemistry and Plasma Processing Laboratory, University of California, Los Angeles. |
| For information, please contact Professor Robert F. Hicks |
| Last Modified May 21, 2007 05:58 PM |