Many therapeutic and diagnostic techniques in medicine depend on interactions of light with tissues. In most cases, radiation is incident on the surface of the tissue and the measured reflectance and transmittance provides information about the medium. Numerous models have been developed that predict the distribution of light in tissue. Radiative transport through skin plays a very important role in all these applications as light must first pass through the skin before it reaches other parts of the body. It is also important when skin is the site for photobiologic reactions to treat various disorders.
The skin is the largest and most accessible organ of the body. Thus, it is suitable for non-invasive sensing. For this purpose, the problem of light transport in a multilayer slab of skin exposed to collimated laser light is solved using the modified method of characteristics. For validation purposes, transient light transport in a homogeneous slab was first simulated.
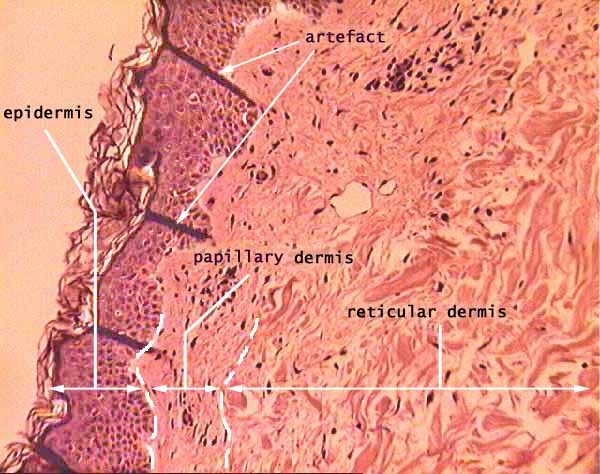
multilayer structure of human skin
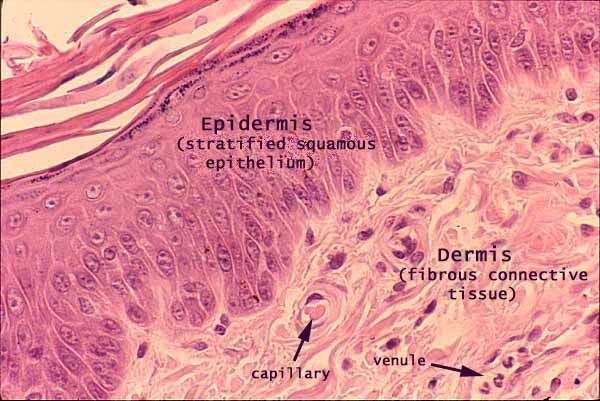
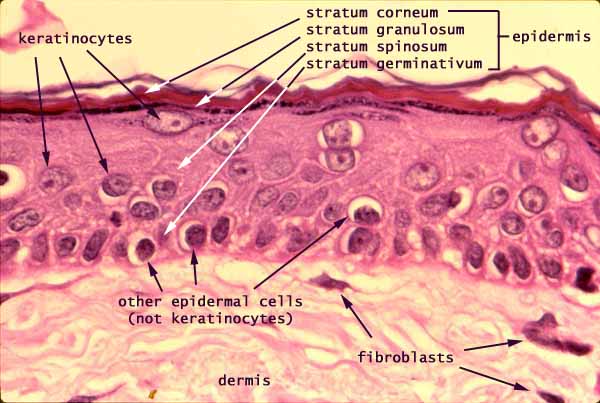
Epidermis and dermis
Experimental Apparatus
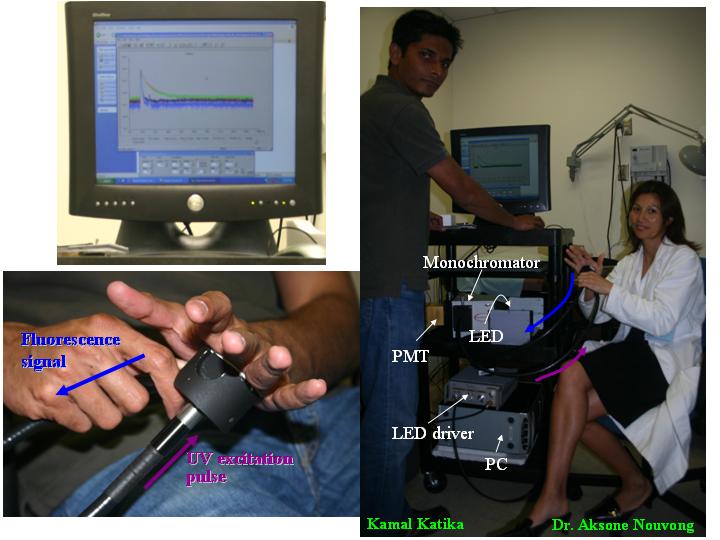
A non-invasive time-resolved fluorescence device has been assembled in our lab and is schematically described in the Figure below. It uses the time-domain technique based on Time Correlated Single Photon Counting (TCSPC). It consists of
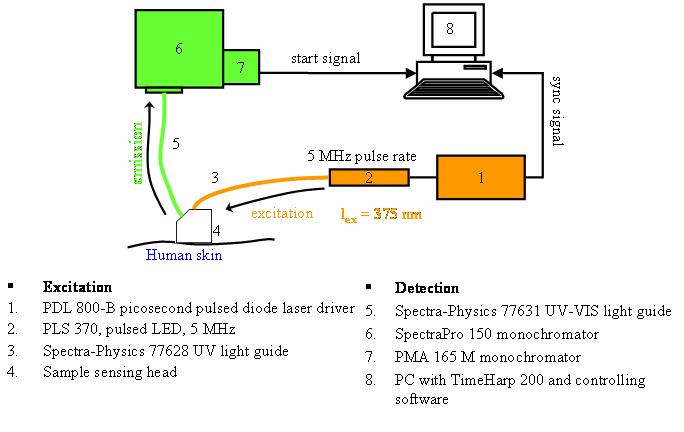
-
UV liquid light guide units (Models 77631 and 77628, Spectra-Physics, U.S.A) attached to the sensing head to channel excitation and emission light,
-
a sensing head or probe constructed to simultaneously excite the sample and collect the resulting fluorescence emission of an area of skin having 2 cm in diameter,
-
an ultra-short pulsed light emitting diode (PLS 370 diode, PicoQuant GmbH) as the excitation source at 375 nm whose pulse width at a repetition rate of 2.5 MHz and average power of 4.8 µW was 664 ps,
-
its associated LED driver at a repetition rate of 5 MHz (PDL 800-B, PicoQuant GmbH),
-
a monochromator (SpectraPro-150, Acton Research Corporation, U.S.A.),
-
a photomultiplier tube (PMT) assembly (PMA-M 165 from PicoQuant GmbH) coupled to the monochromator, and
-
a personal computer (PC).
For the data acquisition, the “sync” output signal from the diode driver and the “start” signal from the PMT assembly were fed to the respective channels on the data acquisition board via standard 50 Ohms coaxial cables. The “sync" output from the diode driver provided the signal to synchronize the timing electronics on the data acquisition board mounted on the PC. The histograms were collected using the TimeHarp software. The first liquid light guide unit transports excitation light pulses to the skin of a patient, while the second receives and transports the reflected and fluorescence signals from the patient to the detector. The excitation ultraviolet pulses will be repeatedly shined onto the skin at a rate of several millions times per second. The successive signal will be added thus increasing the signal to noise ratio and the overall quality and reliability of the signals.
Sample Measurements
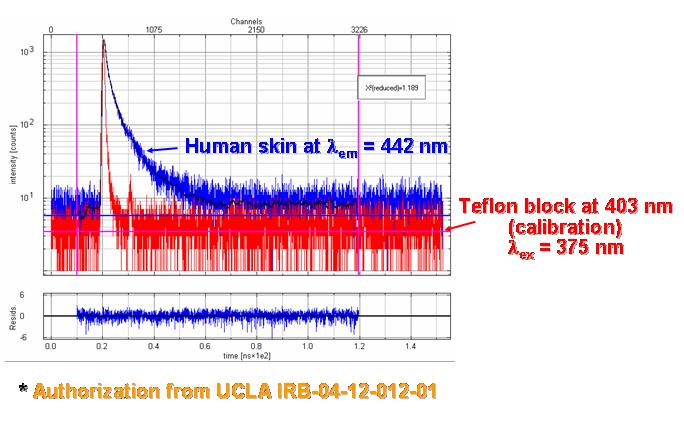
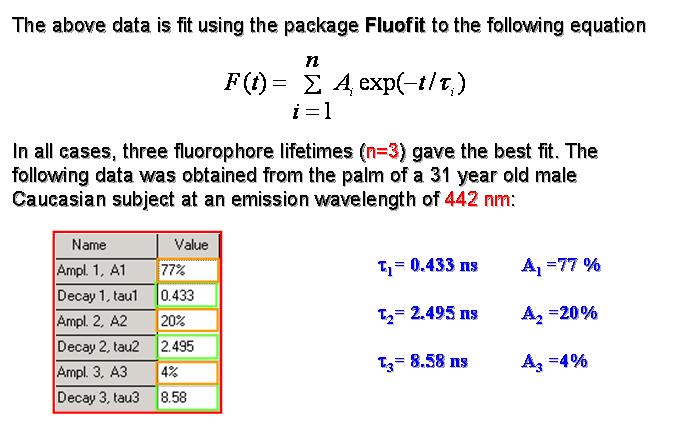
J. Blackwell, K. M. Katika, L. Pilon, K. Dipple, A. Nouvong, S. Levin, and H. Berberoglu, 2008. In-vivo Time-Resolved Autofluorescence Measurements on Human Skin, Journal of Biomedical Optics, Vol. 13, no.1, 014004. doi:10.1117/1.2830658 pdf
K. M. Katika and L. Pilon, 2007. Feasibility Analysis of an Epidermal Glucose Sensor Based on Time-Resolved Fluorescence, Applied Optics, Vol. 46, No.16, pp. 3359-3368. Selected to appear in (i) The Virtual Journal for Biomedical Optics, Vol. 2, No. 8, 2007, (ii) Virtual Journal of Biological Physics Research, Vol. 13, No. 12, 2007. doi:10.1364/AO.46.003359 pdf
K. M. Katika and L. Pilon, 2006. Steady-State Directional Reflectance and Fluorescence of Human Skin, Applied Optics, Vol. 47, No.17, pp.4174-4183. Selected to appear in The Virtual Journal for Biomedical Optics, Vol. 1, No. 7, 2006. doi:10.1364/AO.45.004174 pdf