Abstract
This study presents a theoretical framework developed from first principles for predicting the spatiotemporal thermal behavior of hybrid pseudocapacitors under galvanostatic cycling. It accounts for irreversible and reversible heat generation in the electrolyte and in the electrodes due to Joule heating, electric double layer (EDL) formation, and redox reactions. Detailed numerical simulations were performed to investigate the different local heat generation rates and the temperature as functions of time and cycling current. Numerical predictions showed good qualitative agreement with the limited experimental data available in the literature. Such numerical simulations can be used to physically interpret experimental measurements. Indeed, the present results suggest that a distinctive endothermic peak observed in the experimental heat generation rate resulted from cation starvation in the electrolyte reducing the faradaic current density. In addition, heating due to EDL formation significantly affected the local temperature and must be accounted for. The thermal model and the present results will help define safe modes of operation and develop thermal management strategies for pseudocapacitors.
Highlights
- New thermal model for hybrid pseudocapacitors was rigorously derived from first principles.
- The model accounted simultaneously for faradaic reactions and EDL formation.
- EDL formation was found to significantly influence the thermal behavior.
- Simulations qualitatively reproduced experimentally measured heat generation rates.
Analysis
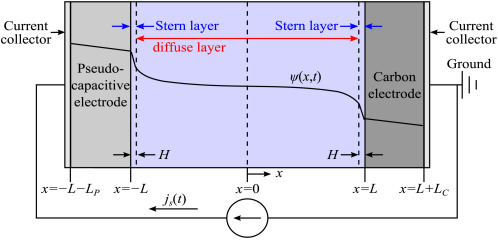
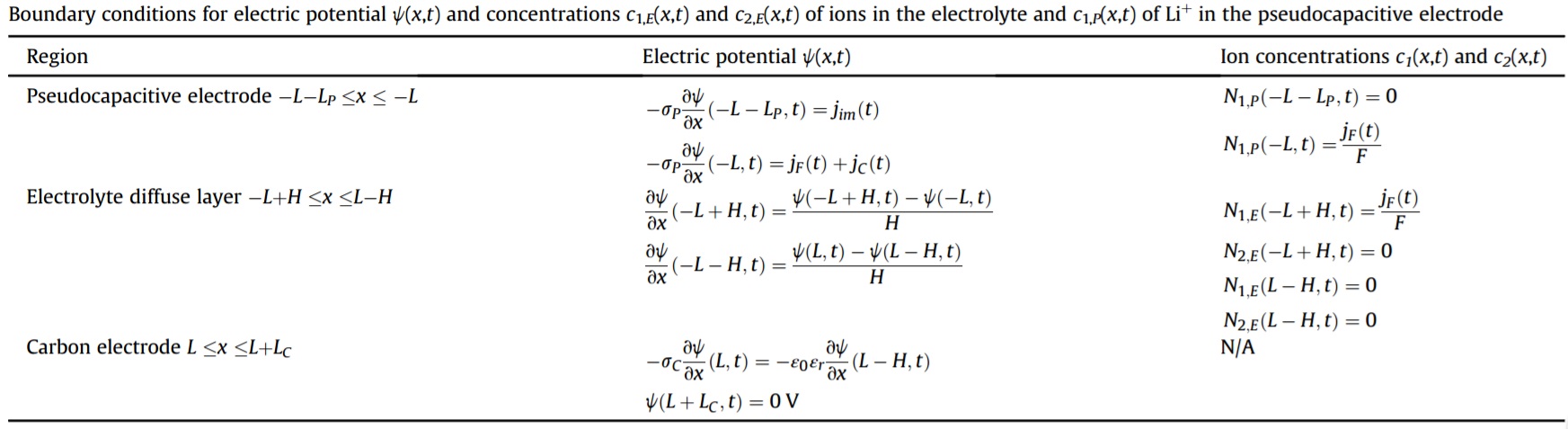
Schematic and Assumptions
Assumptions:
|
|
Governing equations
One-dimensional governing equations for electric potential ψ(x,t), ion concentrations ci,E(x,t), and reduced Li+ concentration c1,P(x,t) in the pseudocapacitive and carbon electrodes and in the electrolyte:
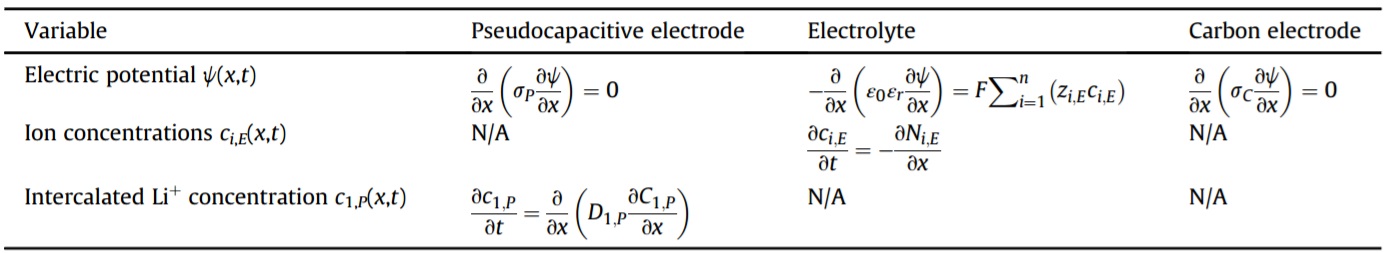
The flux Ni,E of ions in the diffuse layer of the electrolyte is expressed, in 1D, as
Conservation of energy shows that the local temperature T(r,t) is governed by the heat diffusion equation expressed as
![]()
The electrical heat generation rate reduces to irreversible Joule heating expressed as

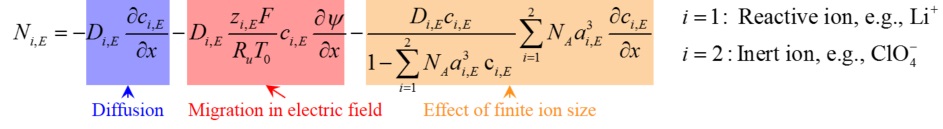
The heat of mixing due to the 1D electron flux and to the 1D flux of intercalated could potentially produce a reversible heat generation rate in the electrode expressed as
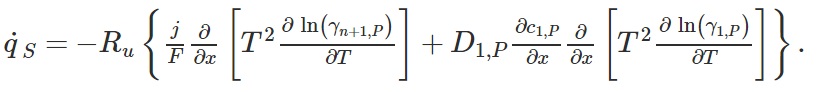
The reversible diffusion and steric contributions are expressed as
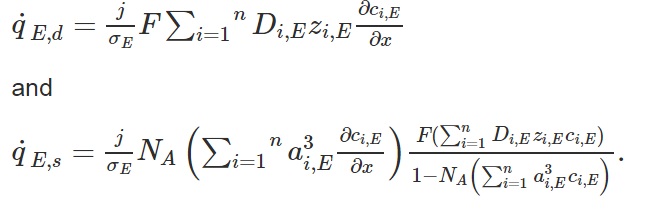
The heat of mixing comprises two terms expressed as
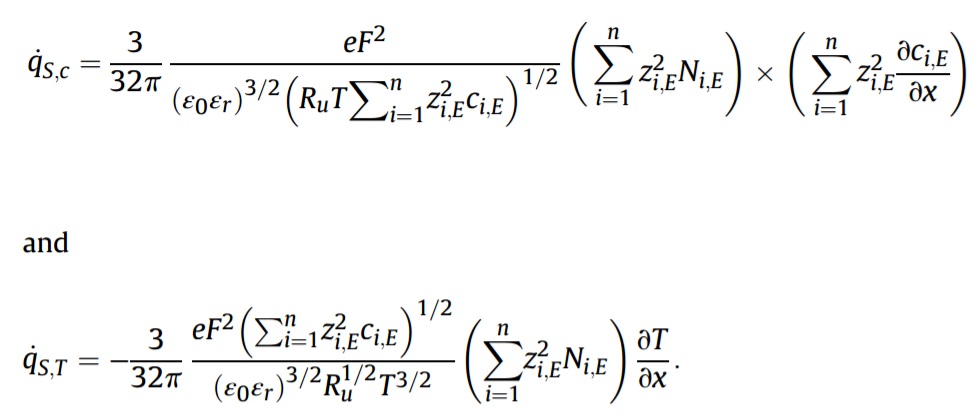
The heat generation rate due to the faradaic reaction is given by
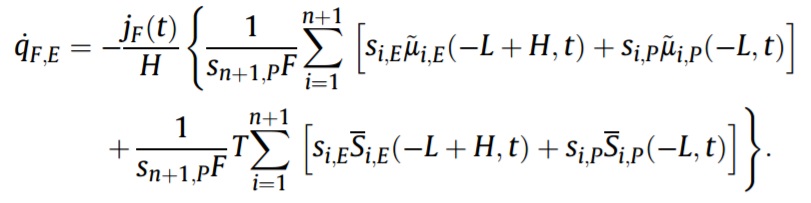
Then, heat generation rate due to the faradaic reaction can be divided into one irreversible and one reversible contribution with the remarkably simple expressions
![]()
Results
Numerically predicted total heat generation rates (a) Q̇P of the pseudocapacitive electrode half-cell and (b) Q"̇C of the carbon electrode half-cell for 0 V ≤ ψs ≤ 0.45 V as well as experimentally measured total heat generation rates minus Joule heating (c) Q̇C-Q̇j,irr,P of the pseudocapacitive electrode half-cell and (d) of the carbon electrode half-cell for a hybrid pseudocapacitor cycled galvanostatically over the potential window 0 V ≤ ψs ≤ 1.0 V [1] as functions of dimensionless time t/tc for different values of js.
Conclusions
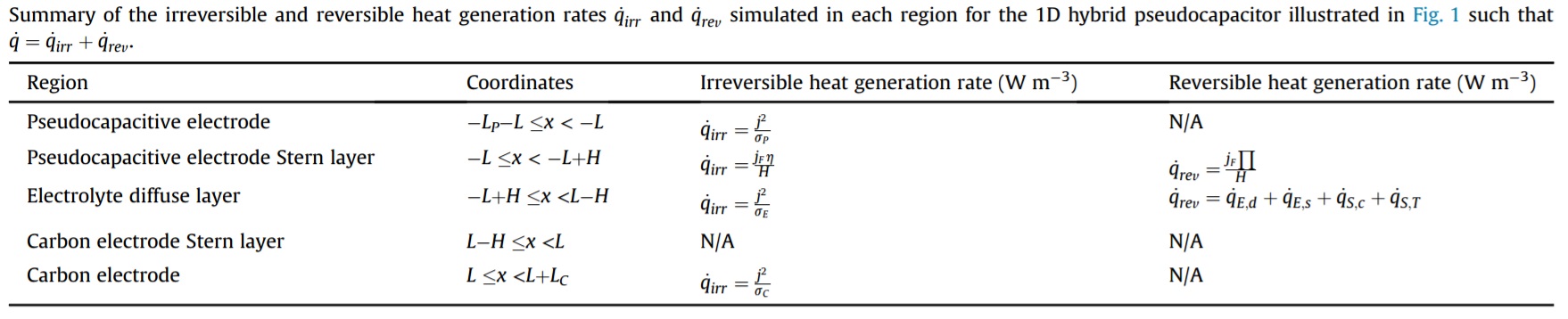
The present study developed the first thermal model based on first principles for the local irreversible and reversible heat generation rates and temperature of EDLCs with multiple ion species and/or asymmetric electrolytes. Detailed numerical simulations were performed for different binary and asymmetric electrolytes based on the properties of aqueous H2SO4. First, the irreversible heat generation rate q̇irr was uniform across the electrolyte and equal to q̇irr =js2/σ∞. It decreased with increasing valency |zi| or diffusion coefficient Di of one or both ion species due to the resulting increase in electrical conductivity of the electrolyte. However, q̇irr was independent of the ion diameter ai. The reversible heat generation rate q̇irr near each electrode was governed by the properties of the counterion. It increased with increasing valency |zi| and decreasing ion diameter ai but was independent of diffusion coefficient Di. As a result, electrolytes with asymmetric valency zi or ion diameter ai featured spatially asymmetric heat generation rates and larger temperature oscillations near the electrode with the larger |zi| or smaller ai of the counterion. The results demonstrate that thermal models must account for electrolyte asymmetry in order to accurately predict the local heat generation rates and temperature. This study suggests that to reduce the overall heat generation in EDLCs, electrolytes should feature large bulk concentrations ci,∞ and at least one ion species with large diffusion coefficient. In addition, electrolytes chosen to yield large capacitance via ions with large valency |zi| and/or small diameter ai are likely to feature large reversible heat generation rates generated near the electrode surfaces.
References
[1] J. Schiffer, D. Linzen, D.U. Sauer, Journal of Power Sources 160 (2006) 765-772.
[2] H. Gualous, H. Louahlia, R. Gallay, IEEE Transactions on Power Electronics 26 (11) (2006) 3402-3409.
Publications
A.L. d'Entremont and L. Pilon, 2014. First Principles Thermal Modeling of Electric Double Layer Capacitors Under Constant-Current Cycling. Journal of Power Sources, Vol. 246, pp. 887- 898. doi:10.1016/j.jpowsour.2013.08.024 pdf
A.L. d’Entremont and L. Pilon, 2014. Scaling Laws For Heat Generation and Temperature Oscillations in EDLCs Under Galvanostatic Cycling. International Journal of Heat and Mass Transfer, Vol. 75, pp. 637–649. doi: 10.1016/j.ijheatmasstransfer.2014.04.001 pdf
A.L. d’Entremont, and L. Pilon, 2014. First-Order Thermal Model of Commercial EDLCs. Applied Thermal Engineering, Vol. 67, pp. 439-446. doi: 10.1016/j.applthermaleng.2014.03.061 pdf
A.L. d’Entremont and L. Pilon, 2015. Thermal Effects of Asymmetric Electrolytes in Electric Double Layer Capacitors. Journal of Power Sources, Vol. 273, pp. 196-209. doi. 10.1016/j.jpowsour.2014.09.080 pdf