Motivations
Microorganisms that are found in nature are not always subjected to optimum illumination. Therefore, as a survival mechanism they have adapted to produce relatively large amounts of pigments. This maximizes the probability of capturing and utilizing photons at low light intensities. However, when these microorganisms are mass cultured in photobioreactors, they absorb more photons than they can utilize and waste the light energy as heat and fluorescence. The emitted fluorescent light can be absorbed by the suspension. However, the fluorescence quantum yield, defined as the ratio of the number of photons emitted by fluorescence to the number of photons absorbed, is typically a few percent. In addition, light does not penetrate deep into the photobioreactor. Thus, the quantum efficiency of photobiological fuel production decreases. Moreover, high intensities can catalyze formation of harmful oxides that can damage the photosynthetic apparatus, a process known as photo-oxidation. Therefore, it is desirable to decrease the chlorophyll antenna size down to the size of the core antenna.
Melis and co-workers physiologically reduced the pigment content of the green algae Dunaliella salina from 1x109 chlorophyll molecules per cell (Chl/cell) to 0.15x109 Chl/cell. More recently, Polle et al.(2003) genetically engineered the green algae \textit{Chlamydomonas reinhardtii} with truncated light harvesting chlorophyll antenna size. The authors reported that the microorganisms with less pigments had higher quantum yield, photosynthesis rate, and saturation fluence rate.
Figure 1 shows the in vivo differential interference contrast (DIC) and chlorophyll fluorescence micrographs of green algae C.reinhardtii CC125 and its truncated chlorophyll antenna transformants tla1, tlaX, and tla1-CW+. It was obtained with a confocal scanning laser microscope in the transmission and epi-fluorescence mode simultaneously at excitation wavelength of 543 nm and fluorescence emission above 560 nm. The figure illustrates the size and shape of each strain as well as the location of the chlorophyll pigments which fluoresce in red. The strong red fluorescence observed in the wild strain CC125 qualitatively shows that it has the highest concentration of chlorophyll while tlaX has the lowest.
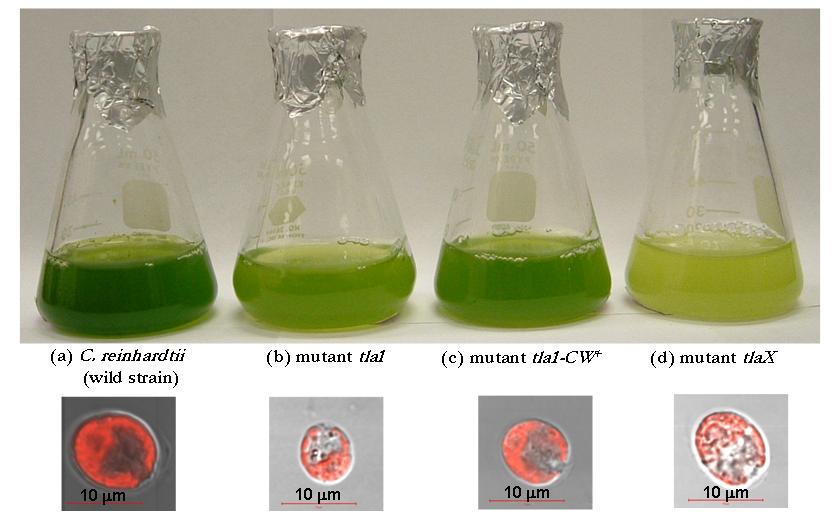
Figure 1. (top) Photograph of flasks containing suspensions of the same concentration of C.reinhardtii (a) CC125, (b) tla1, (c) tlaX, and (d) tla1-CW+ and (bottom) Differential interference contrast and fluorescence micrographs of these green algae. The scale bars correspond to 10 mm.
Radiation Characteristics of C.reinhardtii and its truncated and Its Truncated Chlorophyll Antenna Transformants
Figure 2 shows the absorption and scattering cross-sections of wild and genetically engineered strains of C.reinhardtii between 350 and 750 nm. It is evident that genetically engineered strains have significantly smaller absorption cross-sections than the wild strain. This is particularly true of the mutant tlaX due to their smaller chlorophyll b content. For all mutants, however, the reduction in the absorption cross-section is accompanied by an increase in scattering cross-section. Although scattering becomes the dominant phenomenon contributing to the overall extinction of light, it is mainly in the forward direction so light penetrates within the reactor.
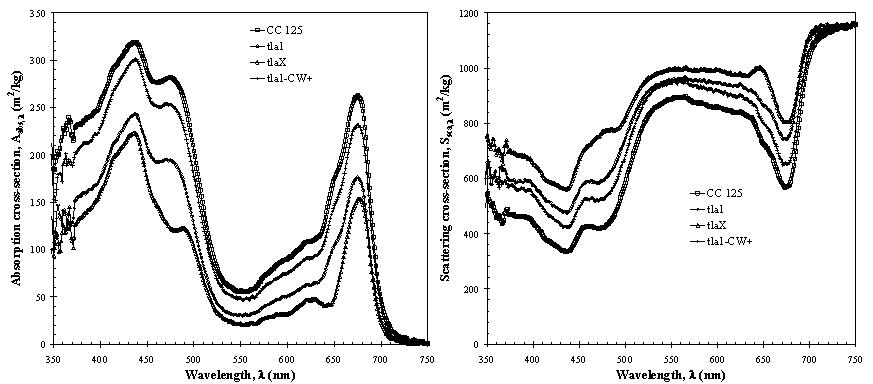
Figure 2. Absorption and scattering cross-sections of the green algae C.reinhardtii CC 125 and its truncated chlorophyll antenna transformants tla1, tlaX, and tla1-CW+ (Berberoğlu et al., 2008).
Publications
A. Bhowmik and L. Pilon, 2016. Can Eukaryotic Cells Be Treated as Optically Homogeneous Spheres?, Journal of the Optical Society of America A, Vol. 33, No. 8, pp. 1495-1503. doi: 10.1364/JOSAA.33.001495 (supplementary material: compartment coordinates) pdf
L. Pilon and H. Berberoğlu, 2014. Photobiological Hydrogen Production. Handbook of Hydrogen Energy, S.A. Sherif, D.Y. Goswami, E.K. Stefanakos, A. Steinfeld, Eds., CRC Press, Taylor and Francis, Boca Raton, FL. ISBN-13: 978-1420054477.
H. Berberoğlu, L. Pilon, and A. Melis, 2008. Radiation Characteristics of Chlamydomonas reinhardtiiCC125 and Its Truncated Chlorophyll Antenna Transformants tla1, tlaX, and 37RP1-tla1, International Journal of Hydrogen Energy, Vol. 33, No.22, pp. 6467–6483. doi:10.1016/j.ijhydene.2008.07.071 pdf