In experimental conditions of heterogeneous catalysis, the structure of the catalytic interface is far from that in vacuum at 0 K. This is extremely important since the superficial structure and composition will completely determine the surface reactivity. We embrace this complexity and construct surface phase diagrams at relevant temperatures and pressures, based on DFT calculations, and incorporating in an explicit way the entropic contributions of gas phase species.
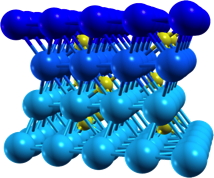
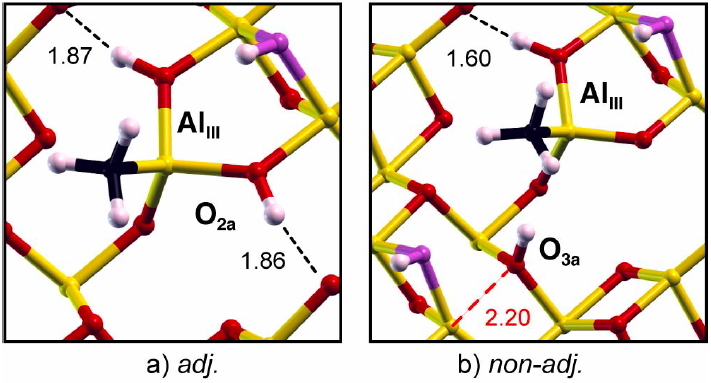
An example of this is the surface of alumina Al2O3, a material heavily used in heterogeneous catalysis. We showed that the relevant surface is in fact hydroxylated. This allows for a detailed understanding of the chemistry of these surfaces and opens numerous applications (grafted complexes or growth of metallic particles on this oxide support).
Another example deals with Pd catalyst for selective hydrogenation of acetylene. We have shown, in collaboration with in situ XPS spectroscopy, that the catalyst surface in conditions of selective hydrogenation is not clean Pd, but a few layer thick film of palladium carbide formed via initial molecular decomposition. The true catalyst is therefore this surface carbide, with strong consequences for the catalytic reactivity and selectivity.