Recent Research Projects
|

|
|
Catalytic conversion of carbon dioxide to liquid fuels and basic chemicals using solar-derived hydrogen at low pressure is a highly desirable goal in heterogeneous catalysis. When realized, this technology will pave the way for a sustainable society together with decentralized power generation. Using high-throughput (HT) catalyst preparation and screening technologies developed in our laboratories, we systematically investigate the oxides of single, binary, ternary and higher-order combinations of metals over differentsupport materials in search for superior catalytic materials.
|

|
|
|
Fabrics of nanofibers synthesized by electrospinning have a broad range of applications such as composite material strengthening, air filtration, tissue engineering, gas sensors, supercapacitors, nanofluidic channels, optoelectronics and more. The fabrication process entails drawing out a solid polymeric fiber from a viscous liquid by applying an electric field. In our work, we use electrospun fibers of metal oxides as catalysts for the oxidative coupling of methane (OCM), the first step in directly utilizing natural gas as an alternate chemical feedstock. Fabrics of La2O3-CeO2 were found to achieve comparable selectivity and yields, for upgrading methane into higher C2+ hydrocarbons, to analogous powder catalysts, except at several hundred degrees Celsius lower feed temperatures. This represents an exciting opportunity in advancing towards catalytic performance that allows OCM to be sustainable economically.
|
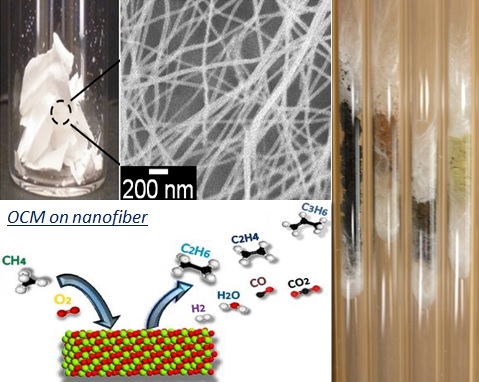
|
|
|
Propylene oxide (PO) is widely used in the preparation of various textiles and plastics with over 8 million pounds of propylene- derived PO produced annually. However, because some of the current production methods result in environmentally hazardous chlorinated byproducts that entail significant costs, the search for an alternative method to achieve a high production rate of PO on an industrial scale has intensified. Although the direct gas-phase epoxidation of propylene to PO by molecular oxygen with the use of heterogeneous catalysts under atmospheric pressure is still the most promising route, it has proven to be a challenging undertaking. Using high-throughput (HT) catalyst preparation and screening technologies developed in our laboratories, we systematically investigate the oxides of single, binary, ternary and higher-order combinations of metals over differentsupport materials in search for superior catalytic materials.
|
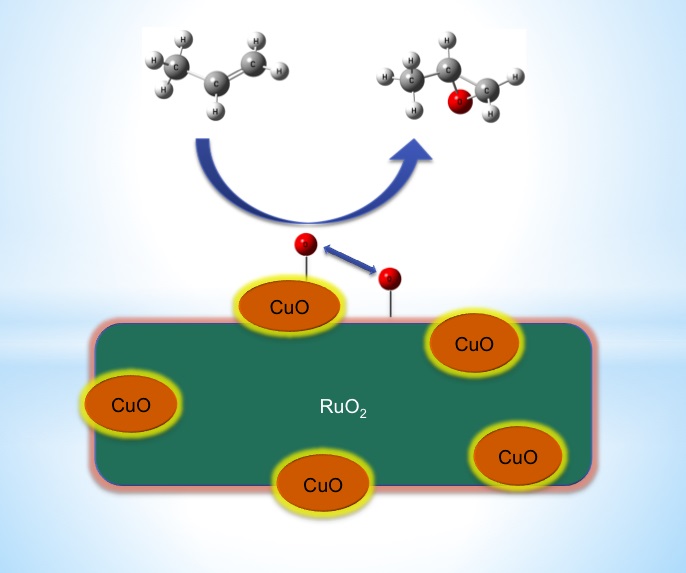
|
|
|
Detailed chemical kinetic mechanism (DCKM) comprise a comprehensive description of chemical transformations in terms of irreducible chemical events or elementary reactions for which independent rate coefficient parameters are available from direct measurements or estimated from theoretical considerations, e.g. density functional theory (DFT) or kinetics descriptors. Once developed, DCKM can be combined with transport models to simulate the behavior of reactors. Therefore, DCKMs represent numerical tools of exceptional generality and broad utility that not only correlate available data but also predict the behavior of complex reaction systems under a very broad range of conditions, including conditions under which the acquisition of experimental data may be impractical, e.g. on the catalyst surface.
|
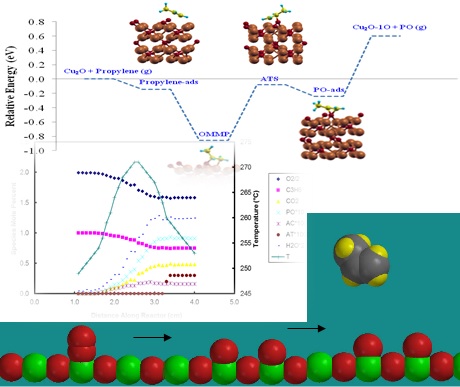
|
|
|
|